CDR proteins: Location, function and interaction
Are CDRs calcium modulators or structural proteins?
About the research project
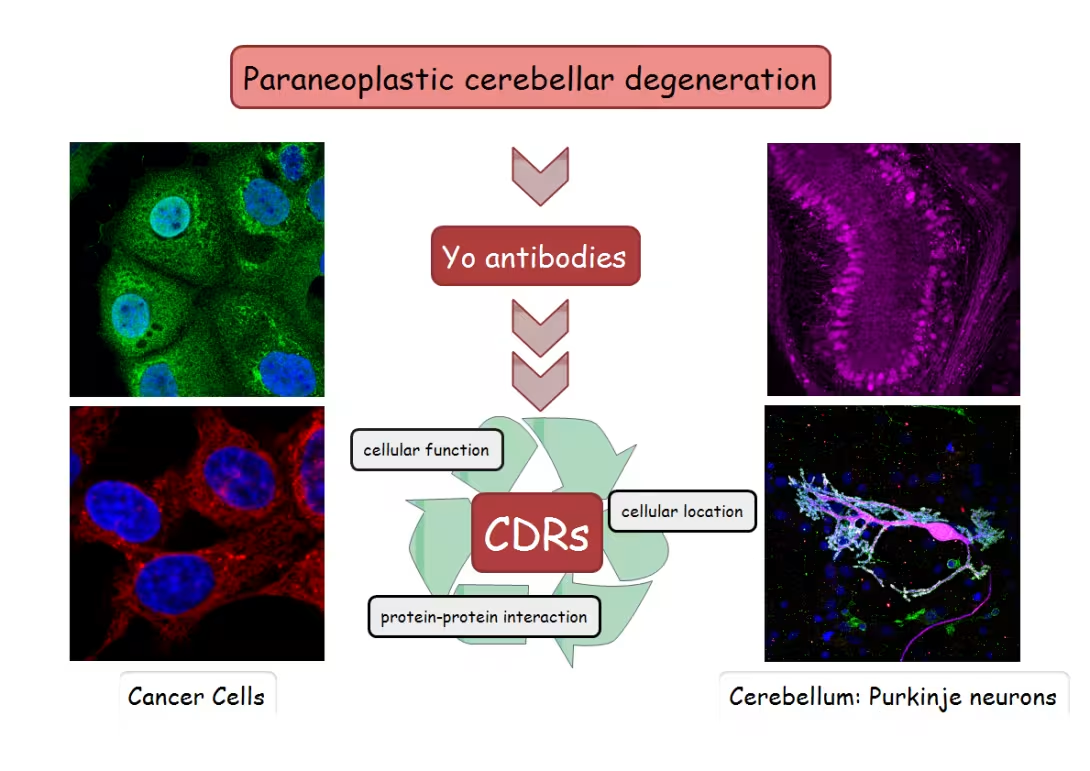
The pathological mechanisms underlying Paraneoplastic Cerebellar Degeneration (PCD) are largely unknown. It is believed that the expression of tumors and genetic alterations of the CDR proteins, especially CDR2L, may trigger an immune response targeting both cancer cells and Purkinje neurons that endogenously express the CDR proteins. However, the mechanisms of immune activation and neural injury induced by these intracellular neural proteins remain unknown. This is partly due to our limited understanding of the biological properties of the CDR proteins.
Our objective is to enhance the understanding of the functional role of CDR proteins, as well as the cellular and molecular mechanisms underlying the death of Purkinje neurons in PCD. Likely, alterations in ion channel functions in the plasma membrane and/or subcellular membranes are the initial key steps in neurological diseases. Growing evidence indicates that both CDR2 and CDR2L play crucial roles in regulating neuronal cell signaling.
To deepen our comprehension of the CDR proteins, we have developed ovarian cancer cell lines in which the CDR1, CDR2, and CDR2L genes have been knocked out using CRISPR/Cas9. Employing a multi-omics approach, we are assessing the effects of the knockout by integrating data from the cells' transcriptome, proteome, and secretome.
Ex-vivo models
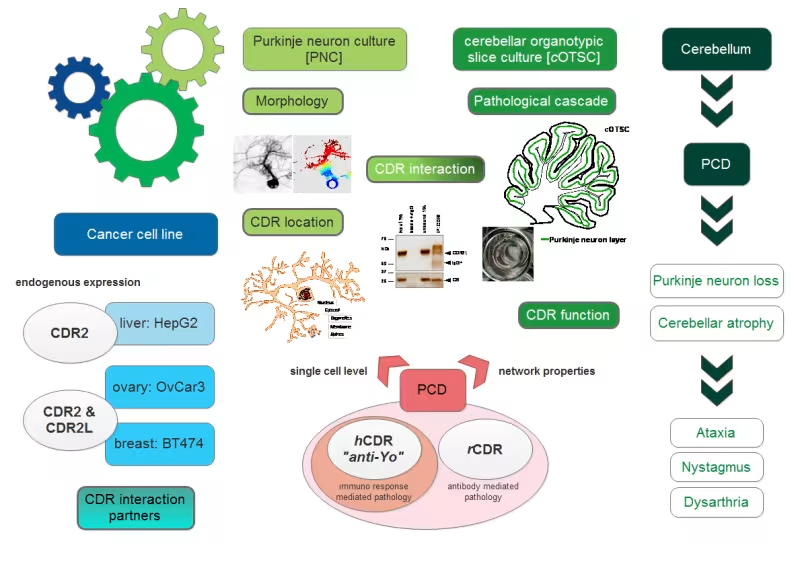
To study the functional role of CDR proteins as well as the pathological role of CDR antibody binding we established three ex-vivo models:
- Cancer cell lines
- Rat Purkinje neuron culture (PNC)
- Rat Cerebellar organotypic slice culture (cOTSC)
Cancer cell lines:
HepG2, OvCar3, OvCar4 and BT474 expressing either both CDR2 and CDR2L or only CDR2 at the endogenous levels. This experimental model system is less sensitive then the neuronal primary culture of Purkinje neurons and it is easy to manipulate by utilizing techniques such as CRISPR-Cas9 to knockout the CDR proteins. We are using these cell lines to study protein location and protein-protein interaction.
Purkinje neuron culture (PNC):
PNC is a heterogeneous primary culture of cerebellar cells with enrichment on Purkinje neurons, the main target in PCD. To obtain a high population of Purkinje neurons can be difficult and exhausting process, because they are extremely sensitive and need additional supplements/nutrients for survival. We mainly use this “single neuronal cell level” model to place/evaluate findings regarding CDR protein location and protein-protein interactions obtained in the cancer cell line studies in neuronal prospective. But also to study anti-Yo and compounds effects on Purkinje neuron morphology.
Cerebellar organotypic slice culture (cOTSC):
OTSC can be used to study neurochemical, structural and physiological changes linked to diseased in vivo brains. Hence, we created two ex-vivo antibody-mediated PCD models by applying anti-Yo (CDR2/2L) positive patient sera (immuno-response and antibody-mediated pathology) and affinity-purified rabbit CDR antibodies (antibody-mediated pathology) to the culture medium of rat cOTSC. These slices have the unique advantages that the tissue architecture is preserved, synaptic circuitries are maintained, and various experimental approaches including compound studies can be evaluated with or without the influence of activated immune cells and the blood-brain barrier regarding PCD pathology.
CDR2 and CDR2L

To develop neuroprotective therapy for PCD patient we have to identify the molecular mechanisms and signaling pathways that are affected after onconeuronal auto-antibody Yo (anti-Yo) pass the blood-brain barrier and bind to cerebellar degeneration-related protein CDR2 and/or CDR2L in Purkinje neurons. To achieve these detailed insights we have to answer some essential questions:
First: What are the functions of both anti-Yo targeted proteins CDR2 and CDR2L in Purkinje neurons?
Very little is known! From a few neurological as well as cancer studies we are confident that CDR2 interacts with calbindin D28K (calcium buffer/modulator); protein kinase N1 (PKN1, signal transduction) and c-myc (nuclear phosphorprotein; cellular transformation), all proteins important for cell survival. Yet, the function/interaction of CDR2L is still undiscovered.
Second: Which biochemical processes are dysregulated when anti-Yo incorporates and binds to CDR2 and/or CDR2L proteins in Purkinje neurons?
To unravel that complexity we have developed different ex-vivo PCD models in the last five years. We discovered that anti-Yo incorporation and binding leads to cellular calcium overload and mitochondrial dysfunction. The activity/function of voltage-gated calcium channels (VGCC), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR), sodium/calcium channels (NCX), mitochondria permeability transition pore (MPTP), protein kinase C gamma (PKCγ), calbindin D28K and calpain-2 was heavily altered dependent on whether CDR2 or CDR2L was the anti-Yo target (DOI: 10.1007/s00401-014-1351-6 (external link) + 10.1111/nan.12492).

