Extracellular vesicles (EVs)
Melanoma has the highest propensity of all cancers to metastasize to the brain, with a large proportion of late-stage patients developing metastases in the central nervous system. Metastasis establishment, cell survival, and progression are strongly influenced by tumor–host cell interactions, with alterations in the host microenvironment playing a critical role.
Published: (Updated: )
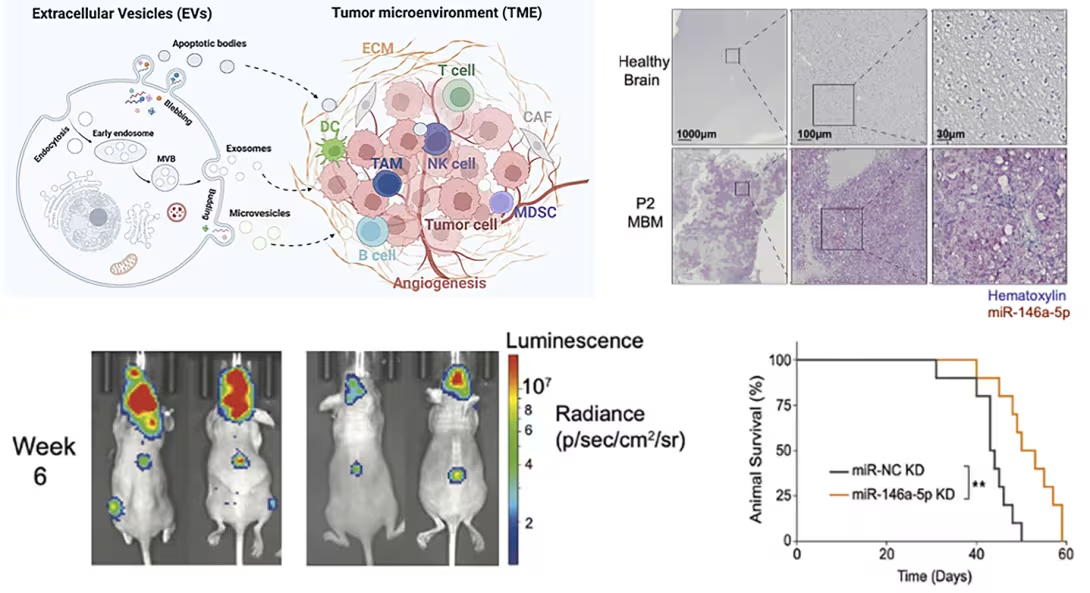
MiRNAs carried by tumor-derived extracellular vesicles (EVs) have been shown to facilitate creation of a pro-metastatic microenvironment. EVs are small vesicles (30–150 nm in diameter) released by all cell types. Circulating EVs derived from cancer cells have been shown to promote metastatic spread, including to the brain.
Our studies (external link) revealed that miR-146a-5p is highly expressed in EVs from human melanoma brain metastases (MBM), both in cell lines and patient biopsies, where it modulates the brain metastatic niche. Mechanistically, miR-146a-5p is transferred to astrocytes via EVs, inhibiting NUMB expression in the Notch signaling pathway. This activates tumor-promoting cytokines including IL-6, IL-8, MCP-1, and CXCL1. Knockdown of miR-146a-5p significantly reduced MBM and lowered cytokine levels in astrocytes.
Through molecular docking analysis, we identified deserpidine as a functional inhibitor of miR-146a-5p, effective both in vitro and in vivo. Our findings highlight the pro-metastatic role of miR-146a-5p in EVs and identify deserpidine as a promising candidate for targeted adjuvant therapy.

