New molecular insights into early protein maturation
Proteins are the workhorses of the cells, and many require processing during their production to achieve proper function. One key player in this process is NatA, an enzyme complex that modifies proteins as they are made. New research now reveals that NatA is more than just a modifying enzyme, it can act as a central hub, coordinating multiple factors during the earliest steps of protein maturation.
By: Nina Mc Tiernan and Thomas Arnesen
Published:
One of the most common protein modifications is N-terminal (Nt) acetylation, which influences protein stability and functionality (external link). An enzyme complex called NatA is responsible for Nt-acetylating around 40% of all human proteins. The core components of NatA are the catalytic subunit NAA10 and the ribosome-anchoring subunit NAA15. In addition, NatA cooperates with other proteins at the ribosome to facilitate protein maturation. Recent research has shown that NatA forms a multi-enzyme complex with the nascent-polypeptide associated complex (NAC) and/or N-terminal methionine excision enzymes (MetAP1 (external link) and MetAP2 (external link)). MetAPs first remove the initiator methionine of a nascent polypeptide if the second amino acid is alanine, serine, valine, glycine, threonine or cysteine, before NatA attaches an acetyl group to the protein’s N-terminus.
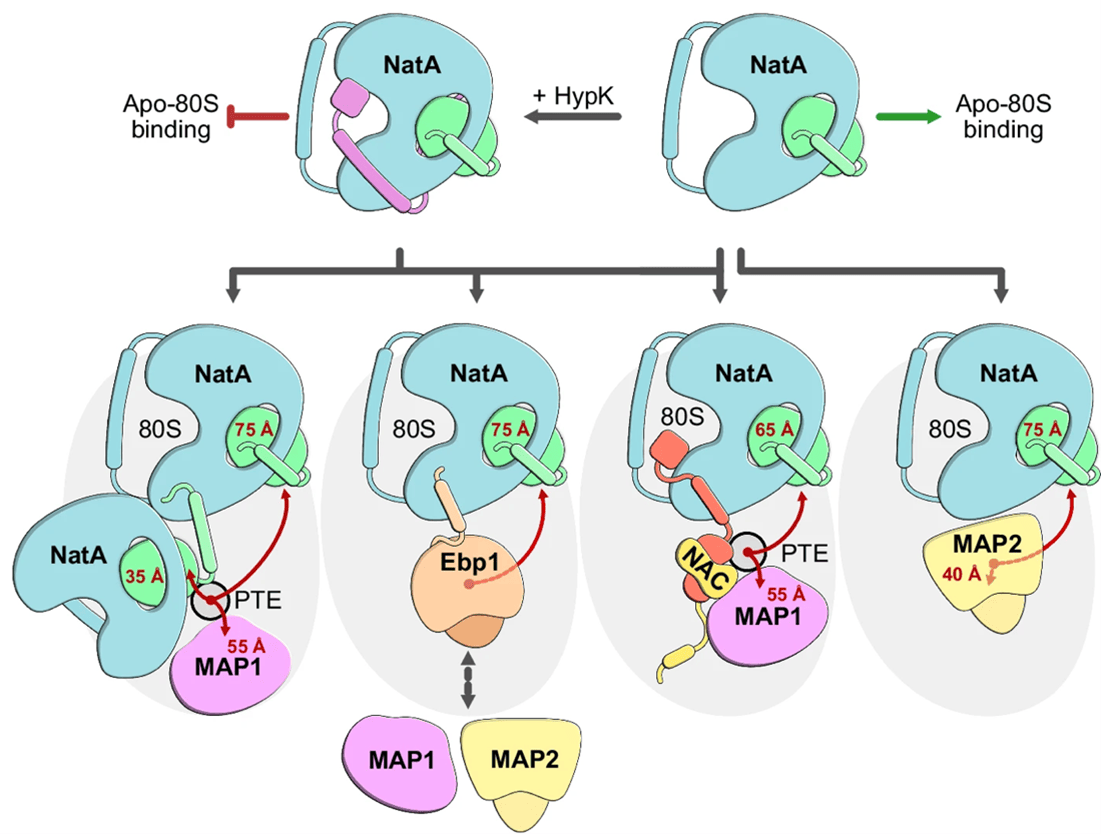
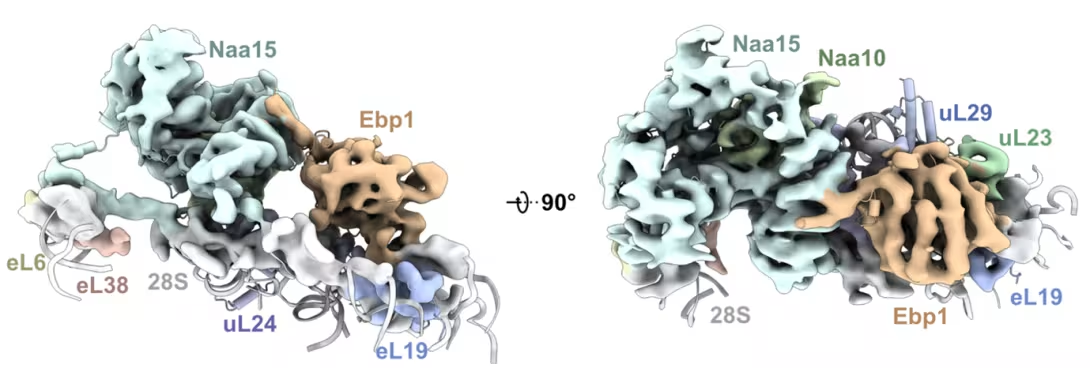
In their most recent work, researchers in Professor Irmgard Sinning’s lab (external link) (Heidelberg University Biochemistry Center) discovered that NatA is even more versatile than previously thought. In vitro binding assays revealed that NatA can also form ribosome-independent complexes with several ribosome-associated factors. Structural data showed that NatA can create a three-part complex with a pseudoenzyme called Ebp1 at the ribosome or even recruit a second NatA complex from a different position near the protein exit tunnel, potentially improving access to its targets. The team also identified a conserved binding site on NAA15 that acts like a docking station for multiple partners, including Ebp1, NAC, NAA10, and HYPK. These interactions enable NatA to assemble different multi-factor complexes at the ribosomal polypeptide tunnel exit. The findings on NatA interactions were supported by experiments in human cells performed by postdoctoral researcher Nina McTiernan in Thomas Arnesen’s group (Department of Biomedicine, UiB).
In sum, this work shows that NatA not only catalyses Nt-acetylation at the ribosome but also functions as an interaction hub coordinating early steps of protein maturation. Gaining insight into these interactions is key to understanding how cells maintain protein quality control.
The published article can be found here:
NatA engages in multi-factor complexes at the ribosomal polypeptide tunnel exit | Nature Communications (external link)