Work Packages
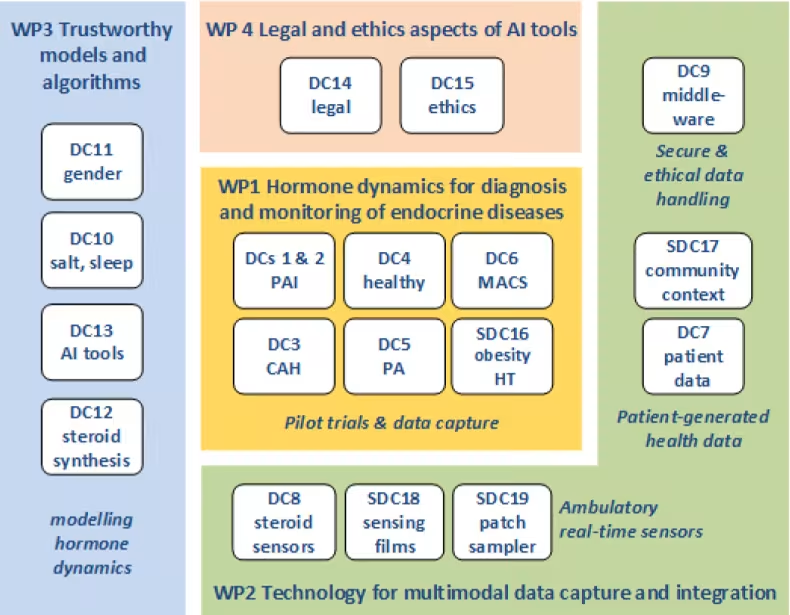
The ENDOTRAIN project is organized into eight Work Packages: four scientific WPs advancing digital endocrinology through clinical studies, technology, modelling, and ethics; and four transversal WPs for training, outreach, management, and compliance—ensuring innovation and integrity.
WP1 – Hormone Dynamics
Lead: LMU
Duration: Months 7–42
Focus: Clinical research to understand hormone rhythms and their role in adrenal disorders.
Details: WP1 investigates dynamic hormone secretion patterns (cortisol, aldosterone) in health and disease using 24-hour profiling combined with wearable data and omics. It aims to improve diagnosis and monitoring of conditions like primary adrenal insufficiency (PAI), congenital adrenal hyperplasia (CAH), primary aldosteronism (PA), and mild autonomous cortisol secretion (MACS). Pilot trials will harmonize protocols across centers, integrating physiological, environmental, and patient-reported data.
Expected Outcomes: New diagnostic criteria, optimized hormone replacement strategies, and concepts for patient self-assessment tools.
WP2 – Technologies for Multimodal Data
Lead: ETHZ
Duration: Months 7–42
Focus: Development of innovative biosensors and data integration tools.
Details: WP2 addresses the technological challenge of capturing real-world, high-frequency data from multiple sources. It includes designing minimally invasive sensors for continuous hormone monitoring (e.g., microneedles, sweat patches), developing platforms for patient-reported data, and creating middleware for secure data handling. Collaboration with WP1 ensures clinical validation of these technologies.
Expected Outcomes: Validated wearable devices, SOPs for responsible data capture, and personalized health recommendation systems.
WP3 – Models and Algorithms
Lead: UBIRM
Duration: Months 7–42
Focus: Mathematical and AI-based modelling of endocrine systems.
Details: WP3 develops mechanistic and hybrid models of the hypothalamic-pituitary-adrenal axis and related systems to predict hormone dynamics and therapy responses. It integrates multimodal data from WP1 and WP2 to build trustworthy algorithms for diagnosis, monitoring, and decision support. AI tools will enable virtual patient simulations and disease classification, ensuring transparency and explainability.
Expected Outcomes: Predictive models, sex-specific simulations, AI-based diagnostic tools, and scalable data infrastructures.
WP4 – Ethics and Legal Aspects
Lead: UiB
Duration: Months 7–42
Focus: Governance framework for digital endocrinology.
Details: WP4 ensures compliance with evolving EU regulations (GDPR, EHDS, AI Act) and embeds ethics-by-design principles throughout the project. It addresses data privacy, fairness, and inclusivity, producing guidelines for responsible use of patient-generated data and AI-driven tools.
Expected Outcomes: Legal analysis of health data management, ethics guidelines, and SOPs for secure data handling.
WP5 – Training
Lead: UiB
Duration: Months 7–48
Focus: Comprehensive doctoral training program.
Details: WP5 delivers interdisciplinary education combining endocrinology, data science, engineering, law, and ethics. It includes network-wide workshops, secondments, and transferable skills training (entrepreneurship, communication, FAIR data practices).
Expected Outcomes: Highly skilled researchers equipped for careers in academia, industry, and healthcare.
WP6 – Knowledge Management & Outreach
Lead: Adhera
Duration: Months 1–48
Focus: Dissemination, communication, and exploitation of results.
Details: WP6 manages outreach to scientific, industrial, and patient communities through publications, conferences, social media, and policy briefs. It also oversees intellectual property strategies and prepares for commercialization of key innovations.
Expected Outcomes: Visibility of project results, stakeholder engagement, and pathways for exploitation.
WP7 – Project Management
Lead: UiB
Duration: Months 1–48
Focus: Coordination and administration of the network.
Details: WP7 ensures smooth implementation of research and training activities, financial management, risk monitoring, and compliance with MSCA guidelines.
Expected Outcomes: Efficient governance, timely deliverables, and strong collaboration across partners.
WP8 – Ethics Requirements
Lead: UiB
Duration: Months 1–48
Focus: Oversight of ethical compliance.
Details: WP8 monitors adherence to ethical principles in all research activities involving human participants, data protection, and AI deployment. It includes ethics reporting and engagement with ethics advisors.
Expected Outcomes: Ethics reports and continuous alignment with EU standards.