SCAN-AED: Nordic register-based study of antiepileptic drugs in pregnancy
SCAN-AED is investigating the association between in utero exposure to antiepileptic drugs and folic acid supplements, and later disease in the mother and child.

About the research project
SCAN-AED is a NordForsk funded Nordic multi-registry study aiming to find the optimal choice of antiepileptic drugs (AEDs) and folic acid treatment during pregnancy. We will examine the risk of physical and neuropsychiatric disease in the child after exposure to different antiepileptic drugs taken alone or in combination during pregnancy. We will also investigate how high dose folic acid modifies this risk. Maternal health during and after pregnancy will also be studied.

Collaboration
CAN-AED is a collaboration between the University of Bergen (Norway), Aarhus University (Denmark), the National Institute for Health and Welfare (Finland), Karolinska Institutet (Sweden), and the University of Iceland.
BIOS (core facility for biostatistics and data analysis, UiB)
The Norwegian Institute of Public Health (the NorPreSS study (external link))
Nordic Microdata Access Network (NordMAN)
Background
Uncontrolled epileptic seizures during pregnancy pose a risk both for the mother and child. Therefore, most women with epilepsy who are using antiepileptic drugs (AEDs) before conception need to do so during pregnancy as well. Unfortunately, many AEDs increase the risk of pregnancy complications and have teratogenic effects. Some AEDs cause major congenital malformations, autism spectrum disorders and impaired development in children exposed in utero. Women with epilepsy are advised to take a dose of folic acid up to 10 times higher than other women, based on the hypothesis that this might reduce the harmful effects of AEDs on the child. However, the efficacy and safety of this treatment strategy is not clear.
Several great joint international efforts have been made to shed light on the adverse effects of AEDs during pregnancy, but there are important unresolved questions due to the lack of statistical power in previous studies. SCAN-AED is a Nordic multi-register study aiming to find the optimal choice of AED and folic acid treatment during pregnancy. More specifically, we aim to examine the risk of physical and neuropsychiatric disease in the child after exposure to different antiepileptic drugs taken alone or in combination during pregnancy. We will also investigate how high dose folic acid modifies this risk. Maternal health during and after pregnancy will also be examined.
The project is a collaboration between epilepsy- and register-based research environments at Aarhus University (Denmark), University of Bergen (Norway), the National Institute for Health and Welfare (Finland) and Karolinska Institutet (Sweden). Data from Iceland will also be included. All five countries have similar population registries that can be linked by the personal identification number. The registers cover almost 20 million people, of whom about 120 000 will have active epilepsy. By linking the medical birth registries with information on maternal medication from the prescription registries, socioeconomic data from the National statistical institutions, and ICD codes for mothers and offspring from the patient registries, we will have an unselected material with very large statistical power that can be adjusted for important confounding factors. We will therefore be able to investigate the association between in utero exposure to various AED regimes and folic acid supplement doses, and later disease in the child and the mother. This study will thereby fill the knowledge gaps in international guidelines for pregnant women with epilepsy.
International conference on antiepileptic drugs in pregnancy and risk for mother and child 2021
In May 2021, SCAN-AED and NorPreSS arranged the “International conference on antiepileptic drugs in pregnancy and risk for mother and child”. The conference was held in a digital format and gathered about 200 participants from 17 countries. Their professional backgrounds spanned from researchers, clinicians, pharmacologists, pharmacovigilance experts, and more. The program included presentations from the patient organization, young researchers, and leading scientists and clinicians in the field. The topics of the different sessions spanned from basic concepts of teratogenicity, methodological aspects, clinical aspects such as pharmacology, neurodevelopment, maternal morbidity and folate supplementation, as well as scientific presentations from SCAN-AED, NorPreSS and others. The conference received excellent feedback in the post-conference survey.
Administrative procedures and personal data protection measures
In May 2021, SCAN-AED and NorPreSS arranged the “International conference on antiepileptic drugs in pregnancy and risk for mother and child”. The conference was held in a digital format and gathered about 200 participants from 17 countries. Their professional backgrounds spanned from researchers, clinicians, pharmacologists, pharmacovigilance experts, and more. The program included presentations from the patient organization, young researchers, and leading scientists and clinicians in the field. The topics of the different sessions spanned from basic concepts of teratogenicity, methodological aspects, clinical aspects such as pharmacology, neurodevelopment, maternal morbidity and folate supplementation, as well as scientific presentations from SCAN-AED, NorPreSS and others. The conference received excellent feedback in the post-conference survey.
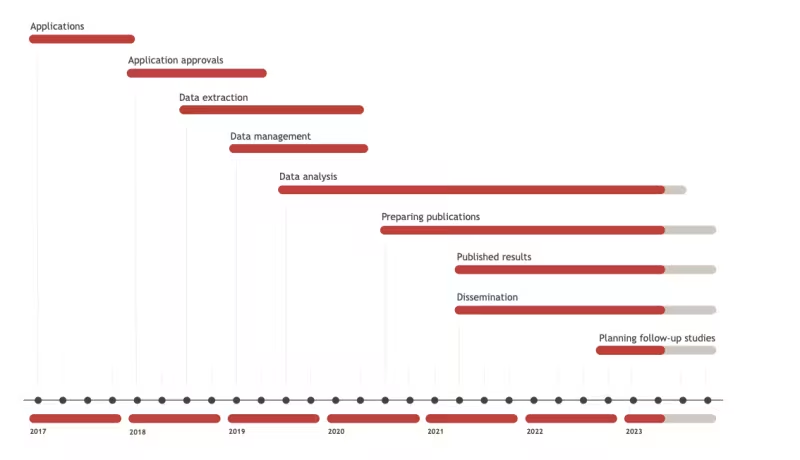
Progress
Financial support
The project is financed by NordForsk (external link)

The group members are also funded by the University of Bergen, Helse Vest/Haukeland University Hospital, The Norwegian Epilepsy Association, Torbjørg Hauge’s legacy, Forsberg and Aulie’s legacy, Karolinska Insitutet, Aarhus Universitetssykehus, Universitetet i Aarhus, National Center for Epilepsy at Oslo University Hospital, and the National Institute for Health and Welfare in Finland.
Publications
Publications in peer-reviewed journals:
Prenatal Exposure to Antiseizure Medication and Incidence of Childhood- and Adolescence-Onset Psychiatric Disorders. Dreier JW, Bjørk MH, Alvestad S, Gissler M, Igland J, Leinonen MK, Sun Y, Zoega H, Cohen JM, Furu K, Tomson T, Christensen J. JAMA Neurol. 2023 Apr 17:e230674. Online ahead of print.
Benefits and Risks of Periconceptional Folic Acid Supplementation-Reply. Vegrim HM, Tomson T, Bjørk MH. JAMA Neurol. 2023 Apr 1;80(4):421-422.
Cancer Risk in Children of Mothers With Epilepsy and High-Dose Folic Acid Use During Pregnancy. Vegrim HM, Dreier JW, Alvestad S, Gilhus NE, Gissler M, Igland J, Leinonen MK, Tomson T, Sun Y, Zoega H, Christensen J, Bjørk MH. JAMA Neurol. 2022 Nov 1;79(11):1130-1138.
Association of Prenatal Exposure to Antiseizure Medication With Risk of Autism and Intellectual Disability. Bjørk MH, Zoega H, Leinonen MK, Cohen JM, Dreier JW, Furu K, Gilhus NE, Gissler M, Hálfdánarson Ó, Igland J, Sun Y, Tomson T, Alvestad S, Christensen J. JAMA Neurol 2022 Jul 1;79(7):672-681.
Prenatal exposure to valproate and risk of congenital malformations—Could we have known earlier?—A population-based cohort study. Christensen J, Trabjerg B, Sun Y, Gilhus NE, Bjørk MH, Tomson T, Dreier JW. Epilepsia. 2021 Sep 28. doi: 10.1111.
Management of epilepsy in pregnancy: a report from the International League Against Epilepsy Task Force on Women and Pregnancy. Tomson T, Battino D, Bromley R, Kochen S, Meador K, Pennell P, Thomas SV. Epileptic Disord. 2019 Nov 29.
Abstracts and presentations:
Prenatal Exposure to Antiseizure Medication and Birth Weight Outcomes. Christensen et al. Platform presentation at the American Epilepsy Society meeting 2022.
Maternal Epilepsy, Prenatal Exposure to High-Dose Folic Acid, and Risk of Childhood Cancer: A Nordic Registry-Based Cohort Study. Vegrim et al. Poster presentation at the American Epilepsy Society meeting 2021.
Folic acid supplementation and risk of neurodevelopmental disorders in children prenatally exposed to anti-seizure medication. SCAN-AED: a Nordic population-based cohort study. Alvestad et al. Poster presentation at the American Epilepsy Society meeting 2021.
Prenatal antiseizure medication exposure and risk of autism and intellectual disability. SCAN-AED: a Nordic cohort study. Bjørk MH, Zoega H, Leinonen MK, Cohen JM, Dreier JW, Furu K, Gilhus NE, Gissler M, Hálfdánarson Ó, Igland J, Sun Y, Tomson T, Alvestad S, Christensen J. Oral presentation at the 7th Congress of the European Academy of Neurology, 19-22 June 2021.
Prenatal exposure to antiseizure medication and the full spectrum of diagnosed psychiatric disorders: A SCAN-AED study. Dreier JW, Bjørk MH, Alvestad S, Gissler M, Igland J, Leinonen MK, Sun Y, Cohen JM, Furu K, Zoega H, Tomson T, Christensen J. Oral presentation at the 7th Congress of the European Academy of Neurology, 19-22 June 2021.
Autism and intellectual disability in children exposed to valproate, lamotrigine, carbamazepine and levetiracetam. Bjørk MH, Zoega H, Leinonen M, Alvestad S, Cohen JM, Dreier JW, Furu K, Gilhus NE, Gissler M, Hálfdánarson Ó, Igland J, Sun Y, Tomson T, Christensen J. Abstract for the 6th Congress of the European Academy of Neurology, 23-26 May 2020.
SCAN-AED summary. Part of a joint position paper from the Danish Epidemiological Society, the Danish Society for Pharmacoepidemiology, and Statistics Denmark, 2019.
Scandinavian multi-registry study of antiepileptic drug teratogenicity: the SCAN-AED study. Bjørk MH, Alvestad S, Gilhus NE, Dreier JW, Christensen J, Gissler M, Leinonen M, Tomson T. Poster at the 5th Congress of European Academy of Neurology, Oslo June 30 – July 2, 2019.
Scandinavian multi-registry study of antiepileptic drug teratogenicity: the SCAN-AED study. Bjørk MH, Alvestad S, Gilhus NE, Dreier JW, Christensen J, Gissler M, Leinonen M, Tomson T. Oral presentation at the 11th NorPEN Meeting, Oslo 7-9 Nov 2018.
People
Project manager
Marte MD PhD
Nils Erik Gilhus MD PhD
Jakob Christensen MD PhD
Mika Gissler Research Professor
Torbjörn Tomson MD PhD
Project members
Silje Alvestad MD PHD
Jannicke Igland PhD
Julie Werenberg Dreier MSPH PhD
Yuelian Sun MD PH
Betina Trabjerg Group member
Neda Razaz PHD

